Overview and Highlights of the Revised Technical Requirements
The U.S.-Canada Greenhouse Certification Program (GCP) Technical Requirements describe the purpose and program requirements of the GCP, the roles and responsibilities of APHIS and CFIA, and the responsibilities of facilities participating in the GCP.
Figure 1 provides a visual overview of the GCP, illustrating how plants enter (Phase 1), are produced (Phase 2), and are certified (Phase 3) through the program.
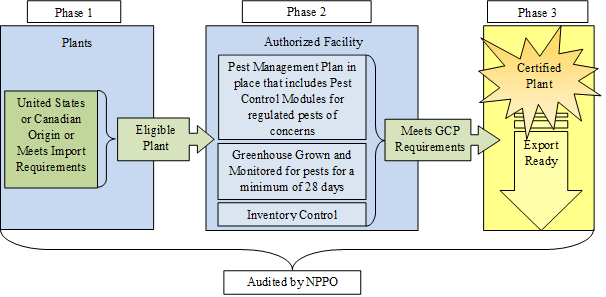
- The GCP is designed to facilitate the certification of plants propagated and grown in a greenhouse.
- When a facility enters into a compliance agreement with its National Plant Protection Organization (NPPO; APHIS or CFIA, as appropriate), it is authorized to produce certified plants. These plants may be exported to the United States or Canada using an Export Certification Label (ECL) in lieu of a phytosanitary certificate.
- ECLs are issued to the authorized facilities by their NPPO. The authorized facility may apply an ECL to the shipping document associated with the certified plants. APHIS and CFIA recognize the ECL as equivalent to a phytosanitary certificate for GCP Certified Plants.
- Certified plants shipped between authorized facilities in the same country may maintain their status through the use of an Interfacility Stamp.
- Facilities maintain records of plant taxa in production and their origins. Only plants that could be imported directly to both Canada and the United States are eligible for GCP certification.
- Facilities have a written pest management plan describing how they monitor and manage plants grown in their greenhouse.
- Facilities are audited by the NPPO according to their compliance agreement to demonstrate that pest risk management measures are functioning as intended and that certified plants consistently meet the requirements of the GCP.
- Authorized facilities in Canada and the United States will be subject to equivalent requirements for participation in the GCP. CFIA and APHIS will coordinate efforts to promote consistent implementation and oversight of the program in both countries.
Highlights
Facilities participating in the current U.S.-Canada Greenhouse Certification Program should review the revised GCP carefully. There are numerous differences from the current programs which may affect the facility. Not all differences are highlighted in this section.
(References to the relevant sections in the GCP Technical Requirements (314.48 KB) are provided)
- The GCP continues to be a program for shipping greenhouse grown plants that are free of regulated pests and practically free of other injurious pests between Canada and the United States using an Export Certification Label in lieu of a phytosanitary certificate.
- A combination of systems and surveillance audits are used to provide regulatory verification that the GCP is being delivered in accordance with the terms agreed to by APHIS and CFIA. (GCP Technical Requirements, Part III, Section 9)
- The revised GCP technical requirements clarify that all plants certified under the GCP, both domestic and foreign-sourced, must meet the phytosanitary import requirements of the United States and Canada. This is not a new requirement, but it is more clearly stated in the revised GCP. (GCP Technical Requirements, Part II, Section I)
- Plant taxa and origin – Authorized facilities are required to maintain a list of plants produced under the GCP and their origins. The NPPO will review the list with the facility to verify that the plants are eligible for the GCP. Guidance is being developed to promote consistent evaluation of these lists. (GCP Technical Requirements, Part III, Section 8.3)
- Certified plants must be free of pests regulated by either the United States or Canada. (GCP Technical Requirements, Part II, Section 5)
- Facilities are required to have a written Pest Management Plan. A template is provided to assist the GCP facility in developing their pest management plan and to provide a standardized format for all GCP participants. (GCP Technical Requirements, Part II, Section 6 and Part V, Appendix I)
- Pest Control Modules – the Pest Management Plan may require detailed procedures (modules) for specific regulated pests. (GCP Technical Requirements, Part II, Section 7.1 and Part V, Appendix II)
- Compliance Agreement – APHIS and CFIA will authorize facilities using equivalent compliance agreements with common elements. (GCP Technical Requirements, Part IV, Section 2)
- Audit Checklist – United States and Canadian auditors will use equivalent audit checklists. (GCP Technical Requirements, Part V, Appendices IV and V)

